2026 AADA Congressional Advocacy Agenda
The American Academy of Dermatology Association advocates on issues and prioritizes work on those that are most pressing and impactful for our membership.
2026 Sole Federal Congressional Advocacy Priority: Protecting Medicare Access to Physicians.
To establish a positive annual inflation adjustment.
To replace or eliminate budget neutrality requirements to the physician payment system.
Download a copy of our agenda
Download a PDF version of our 2026 advocacy agenda.
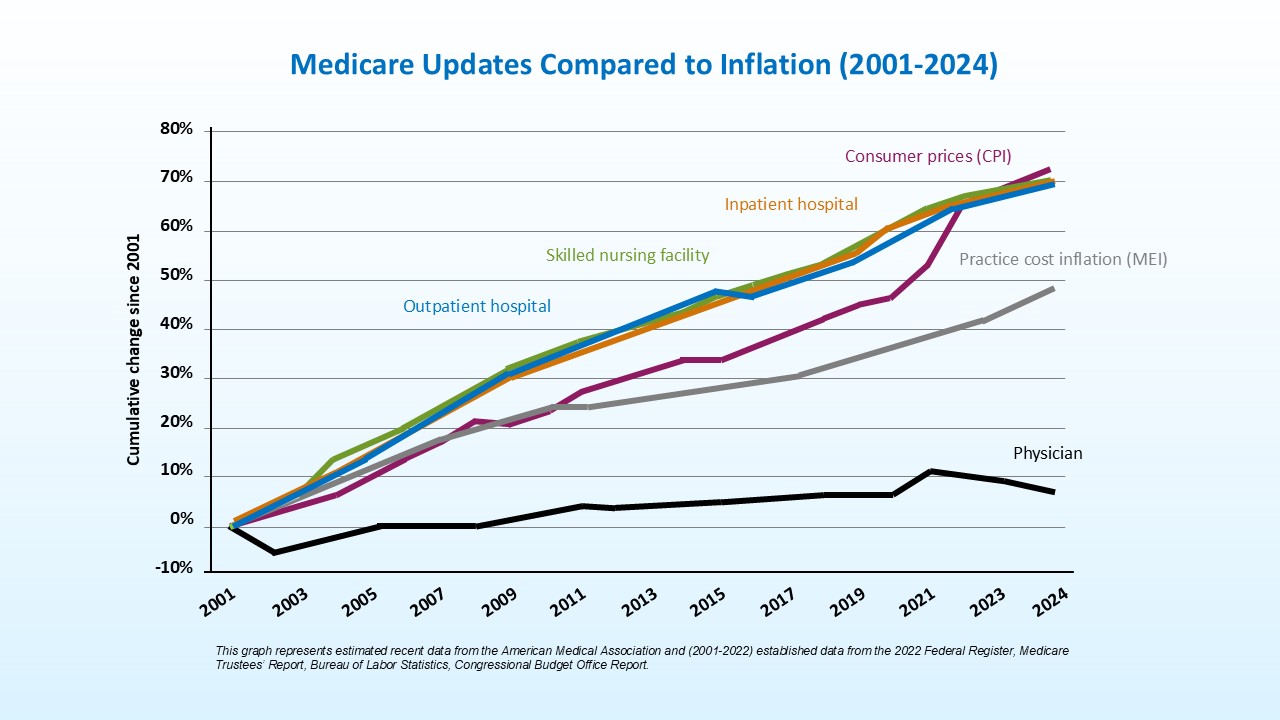
Temporary fixes and continued cuts to the Medicare physician payment system are destabilizing dermatology practices and threatening patient access to care. Since 2001, the cost of operating a medical practice has increased 60%, while economy-wide inflation has risen 83%. During this time, Medicare hospital facility updates increased by roughly 73%, significantly outpacing physician reimbursement. Adjusted for inflation in practice costs, Medicare physician reimbursement has declined 33% from 2001 to 2026.
Congress should provide physicians with positive, inflation-based reimbursement updates to ensure practices maintain financial stability and patients have continued access to care. It is also imperative that Congress revise the budget neutrality policies to: (a) prevent erroneous utilization estimates from leading to inappropriate cuts; (b) clarify the types of services subject to budget neutrality adjustments; and (c) update the projected expenditure threshold triggering the budget neutrality adjustment, which has remained unchanged since 1992.
Medicare physician payment updates
Learn more about the Academy’s ongoing advocacy efforts on Medicare physician payment.
Medicare updatesMake your voice heard
Use our online portal to tell Congress to reform Medicare physician payment rates.
Contact Congress Find a Dermatologist
Find a Dermatologist
 Member directory
Member directory
 AAD Learning Center
AAD Learning Center
 2026 AAD Annual Meeting
2026 AAD Annual Meeting
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Physician wellness
Physician wellness
 Joining or selling a practice?
Joining or selling a practice?
 Promote the specialty
Promote the specialty
 Advocacy priorities
Advocacy priorities
