DermWorld
Dermatologists discuss the possibilities — and pitfalls — of ChatGPT in clinical practice.

Dermatologists discuss the influx of new JAK inhibitors in dermatology and how they are breaking new ground for the specialty.
Pulling back the curtain on PBMs for transparent drug pricing.


Academy coding staff address important coding topics each month.

DermWorld’s physician editor interviews the authors of recent dermatologic studies.

Each month, DermWorld tackles issues “in practice” for dermatologists.

DermWorld covers legal issues in “Legally Speaking.”

Ask the Expert: John Barbieri, MD, MBA, FAAD, discusses important changes to the iPLEDGE program.
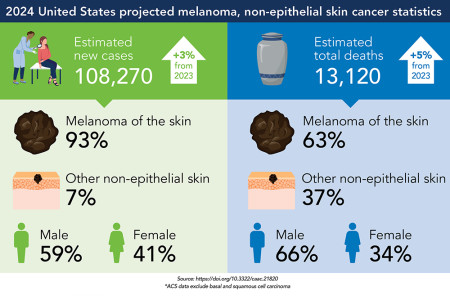
Skin cancer incidence and related deaths projected to rise in 2024.

What has caused it, and what resources can help? Dermatologists discuss solutions.

Recent advances in vitiligo treatment and research open doors for physicians and patients.
Physician editor Kathryn Schwarzenberger, MD, previews this month’s issue.
Members of DermWorld’s Editorial Advisory Workgroup share exciting news from across the specialty.
Academy President Seemal R. Desai, MD, FAAD, discusses his goals for his term as president.
How do I claim CME credit form the AAD Annual Meeting?
 Find a Dermatologist
Find a Dermatologist
 Member directory
Member directory
 2024 AAD Innovation Academy
2024 AAD Innovation Academy
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Physician wellness
Physician wellness
 Joining or selling a practice?
Joining or selling a practice?
 Promote the specialty
Promote the specialty
 Advocacy priorities
Advocacy priorities
