MIPS reporting and fee schedule
CMS delays release of 2024 MIPS final scores
CMS has delayed final 2024 MIPS scores due to incomplete Medicare claims data needed for cost measure calculations. Final scores and the Targeted Review period are now expected in Fall 2025, with payment adjustments for 2026 to follow about a month later.
Explore the Academy’s tools and resources to help you better understand MIPS and the Medicare Physician Fee Schedule. Use our online form to contact practice management staff with questions or concerns.

Access a guide to help you decide whether to participate in MIPS, and how to avoid a penalty and earn an incentive. This efficient, intuitive tool makes choosing your MIPS path much easier.

Access MIPS measures and QCDR measures.

See the Academy’s analysis of MIPS updates for the 2026 reporting year.
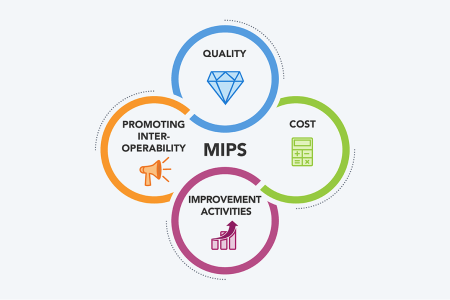
Access the Academy's resources on the four MIPS categories, namely Quality, Promoting Interoperability, Improvement Activities, and Cost.

Learn how this year's Medicare fee schedule will impact reimbursement. Review impacted codes that are most often used by dermatologists.

DataDerm can help with your MIPS reporting requirements. Reach out to the DataDerm team to determine if your EHR integrates with DataDerm at dataderm@aad.org.

Learn more about the Academy's new consumer positioning strategy that reimagines the AAD’s public relations efforts.
 Find a Dermatologist
Find a Dermatologist
 Member directory
Member directory
 AAD Learning Center
AAD Learning Center
 2026 AAD Annual Meeting
2026 AAD Annual Meeting
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Physician wellness
Physician wellness
 Joining or selling a practice?
Joining or selling a practice?
 Promote the specialty
Promote the specialty
 Advocacy priorities
Advocacy priorities
