DermWorld

Dermatologists discuss the cutaneous signs of established and emerging illicit substances.
Experts share best practices and review the latest on safety concerns for anesthesia use in children.
What dermatologists need to know about this year’s MIPS reporting.


Academy coding staff address important coding topics each month.

DermWorld’s physician editor interviews the authors of recent dermatologic studies.

Each month, DermWorld tackles issues “in practice” for dermatologists.

DermWorld covers legal issues in “Legally Speaking.”

Moving the needle: FDA agrees to implement AADA’s iPLEDGE recommendations.
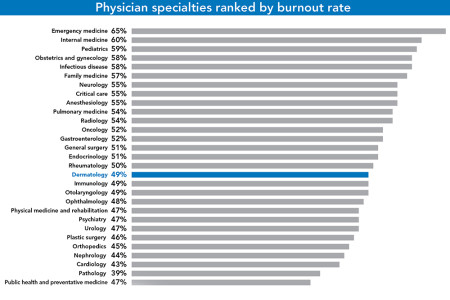
Measuring physician mental health.

DermWorld explores considerations for dermatologists when treating patients experiencing homelessness.

Dermatology hospitalists work in several roles to deliver quality expert care.
Physician editor Kathryn Schwarzenberger, MD, previews this month’s issue.
Members of DermWorld’s Editorial Advisory Workgroup share exciting news from across the specialty.
Academy President Seemal R. Desai, MD, FAAD, offers highlights of his first month in office.
Where can I find the AAD fellow logo and how can use this to promote my practice?
 Find a Dermatologist
Find a Dermatologist
 Member directory
Member directory
 2024 AAD Innovation Academy
2024 AAD Innovation Academy
 AAD Learning Center
AAD Learning Center
 Need coding help?
Need coding help?
 Reduce burdens
Reduce burdens
 Clinical guidelines
Clinical guidelines
 Why use AAD measures?
Why use AAD measures?
 Latest news
Latest news
 New insights
New insights
 Physician wellness
Physician wellness
 Joining or selling a practice?
Joining or selling a practice?
 Promote the specialty
Promote the specialty
 Advocacy priorities
Advocacy priorities
